|
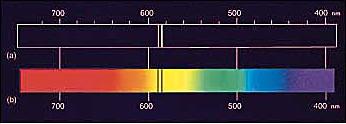
and Spectroscopy |
 |
|
and Spectroscopy |
 |
|
Radiation from Different Types of Sources (Kirchhoff's Laws)
|
|
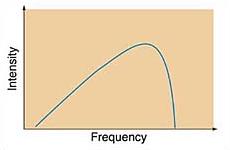
Continuous Spectra: Temperature Effects
|
 |
|
|
Line Spectra: How Can We Explain Them?
|
|
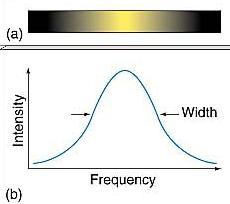
Line Spectra: What Additional Factors Affect Them?
|
 |
 |
|
Other Types of Radiation; Conclusion
|
![]()
page by luca bombelli <bombelli at olemiss.edu>, modified 29 sep 2012